Abstract:
As per International Energy Agency (IEA) reports more than 60% of the vehicle sold will be EVs only by 2030. The increasing demand for lithium-ion batteries (LIBs) in EVs has led to a surge in their production, usage, and disposal. However, improper disposal of these batteries can lead to environmental pollution and resource depletion, making the recycling of lithium-ion batteries a critical issue. This literature review aims to provide an overview of the various recycling methods of LIBs, including pyrometallurgical, hydrometallurgical, and direct recycling. This paper compares the relative advantages and disadvantages of each method. It also discusses the current challenges and potential opportunities for these methods in near future. We conclude with the future work which includes improving the efficiency, sustainability, and cost-effectiveness of the processes while minimizing the environmental impact. Additionally, the recovery of other valuable metals and the development of a circular economy for Lithium-ion batteries could also be potential future directions.
Keywords: – Electric Vehicles (EVs); Lithium Ion Battery (LiB);
Introduction
The demand for electric vehicles (EVs) is rising because of the growing number of regulations imposed on decarbonization in the transportation sector. Due to its high volumetric and gravimetric energy density, lengthy calendar life, low maintenance requirements, and capacity for high power, lithium-ion batteries (LIBs) are the most common energy storage technology used to power EVs. As a result, LIBs can satisfy the user’s requirements for high torque and speed as well as range. The demand for EVs is anticipated to rise further in the upcoming decade, which would lead to an exponential rise in lithium-ion market output. As a result, the demand for raw materials will increase and worries about the supply of essential elements, particularly lithium (Li) and cobalt (Co), will intensify. So, in section1 we will see about the different components and schematics of Lithium Ion Battery, Section 2 will give about different recycling methodologies available. In section 3 we will cover about different advantages and disadvantages of various method. In section 4 we will see about the current challenges and Opportunities in implementing the existing method.
1. Lithium Ion battery overview
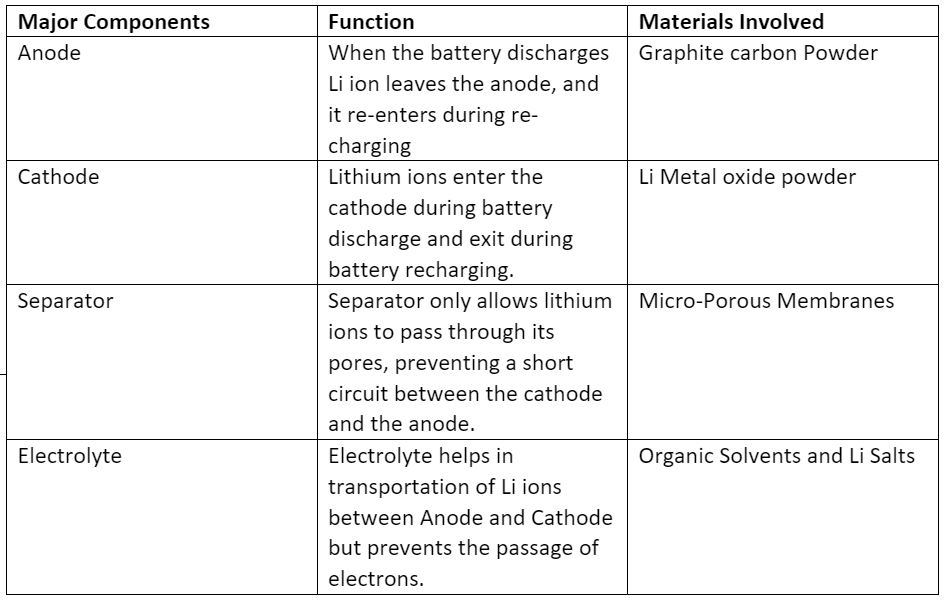
Lithium Ion Battery has four major components like Cathode, Anode, Separator and Electrolyte. Table 1 shows the major components of Li battery along with their function and the common materials involved in their preparation. The electrode materials in lithium-ion batteries are typically composed of lithium and graphite for the anode materials. It also has a layered structure on a copper current collector, and lithium metal oxide for the cathode materials, which either have a layered or tunnelled structure or on an aluminium current collector. A lithium-ion battery charges by transferring lithium ions from the cathode to the anode, while discharging involves the transfer of lithium ions from the anode to the cathode. During charging, an external power source applies a voltage to the battery, causing lithium ions to move from the cathode to the anode through an electrolyte solution. During discharging, the battery is connected to a load, and the flow of lithium ions from the anode to the cathode creates an electrical current that can be used to power the device.
The schematic diagram of electrochemical reactions in Lithium-Ion cell is shown in fig 1.
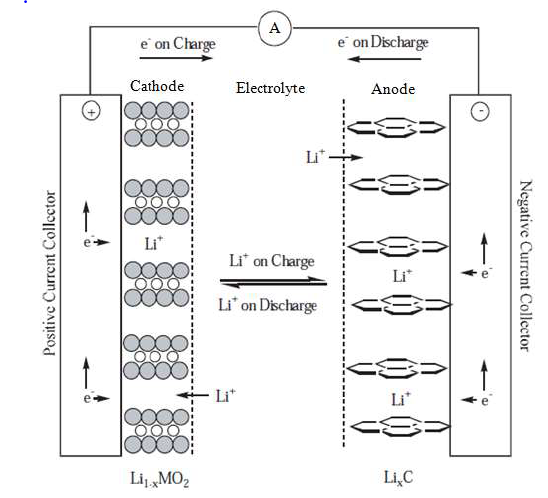
Fig.1 Schematic diagram of electrochemical reaction in Li cell
Table 1: Major components of Lithium-ion battery
2. Methodologies used for LIB Recycling and Industrially Applicable Techniques
There are several methodologies used for LIB recycling and each has its own unique advantages and disadvantages. Here we will discuss the commonly used methodologies and industrially applicable techniques for LIB recycling. In Fig 2 it shows the various recycling methods available and how it is being carried out.
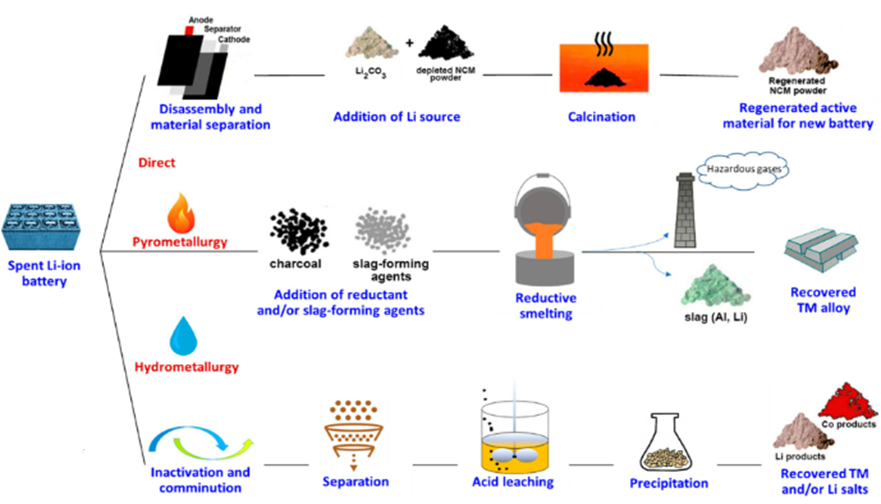
Fig 2. Schematic diagram of Recycling Method
Hydrometallurgical processes
Hydrometallurgical processes are one of the primary methods used to recover metals from spent lithium-ion batteries. This process involves the use of aqueous solutions to dissolve and separate the valuable metals from other battery components, such as plastics and electrolytes.
The hydrometallurgical process typically involves several steps, including leaching, purification, and recovery. Here is a brief overview of each of these steps:
1. Leaching: In this step, the spent batteries are crushed or shredded and then subjected to a leaching process. The leaching solution may be acidic, alkaline, or a combination of both, depending on the type of battery and the metals that need to be recovered. The leaching process dissolves the metals into solution, leaving behind the plastics, electrolytes, and other non-metallic materials.
2. Purification: Once the metals have been dissolved into solution, the next step is to purify the solution by removing any impurities. This can be achieved using various techniques, such as solvent extraction, ion exchange, or precipitation. Solvent extraction involves using an organic solvent to selectively extract the target metals from the leaching solution. Ion exchange involves using a resin to selectively bind and remove the target metals from the solution. Precipitation involves adding a chemical reagent to the solution to cause the metals to precipitate out as solids.
3. Recovery: Once the metals have been purified, the final step is to recover them from the solution. This can be achieved using techniques such as electrowinning, precipitation, or crystallization. Electrowinning involves passing an electric current through the purified solution to selectively plate out the target metals onto an electrode. Precipitation and crystallization involve adding a chemical reagent to the solution to cause the metals to precipitate out as solids.
Pyrometallurgical processes
Pyrometallurgical processes are another method used for recycling lithium-ion batteries. These processes involve the use of high-temperature furnaces to melt and separate the various components of the battery, including metals, plastics, and electrolytes.
The pyrometallurgical process typically involves several steps, including smelting, refining, and casting. Here is a brief overview of each of these steps:
1. Smelting: In this step, the spent batteries are first crushed or shredded and then subjected to high temperatures in a smelter furnace. The high temperatures cause the metals to melt and separate from the other components of the battery. During the smelting process, the electrolytes and other non-metallic materials are burned off or converted to slag.
2. Refining: Once the metals have been separated from the other battery components, they are further refined to remove any impurities. This can be achieved using techniques such as electrorefining or vacuum distillation. Electrorefining involves passing an electric current through the molten metal to selectively remove any impurities. Vacuum distillation involves heating the metal to high temperatures in a vacuum to evaporate and remove any impurities.
3. Casting: Once the metals have been purified, they are cast into ingots or other forms for use in manufacturing new batteries or other products.
While pyrometallurgical processes are an effective way to recover metals from lithium-ion batteries, they can be more energy-intensive and environmentally impactful than hydrometallurgical processes. The high temperatures required for smelting can produce emissions and waste products that need to be carefully managed to avoid environmental contamination. Additionally, the refining and casting steps can require additional energy and resources.
Overall, the choice between hydrometallurgical and pyrometallurgical processes for lithium-ion battery recycling will depend on a variety of factors, including the type of battery, the composition of the materials to be recovered, and the environmental regulations in place. Both processes have their strengths and limitations, and it is important to carefully evaluate the options and work with experienced professionals to ensure the safe and effective recycling of lithium-ion batteries.
Direct Process
Direct recycling processes are a newer method of recycling lithium-ion batteries that has gained attention in recent years. This process involves directly reusing the battery components, such as cathodes and anodes, to manufacture new batteries.
The direct recycling process typically involves several steps, including disassembly, sorting, and reassembly. Here is a brief overview of each of these steps:
1. Disassembly: In this step, the spent batteries are disassembled into their component parts, including the cathode, anode, electrolyte, and separator. The disassembly process may involve manual or automated techniques, depending on the type and size of the batteries.
2. Sorting: Once the battery components have been disassembled, they are sorted and tested to determine their suitability for reuse. This may involve testing the components for their chemical and physical properties, as well as their performance in battery applications.
3. Reassembly: Once the components have been sorted and tested, they can be reassembled into new batteries. This may involve combining the recycled components with new components to achieve the desired performance characteristics.
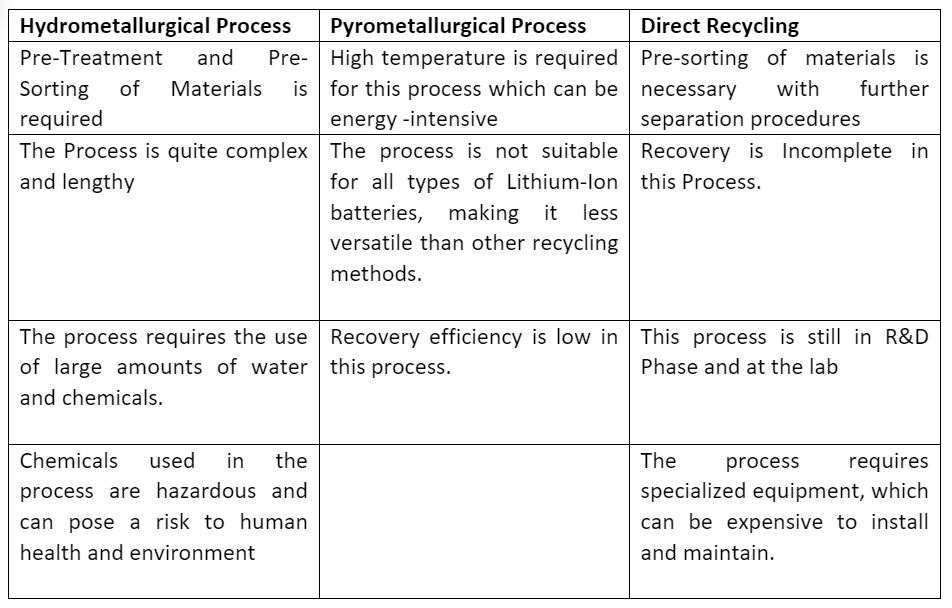
3. Comparison of Hydrometallurgical, Pyrometallurgical and Direct recycling process:
Advantages
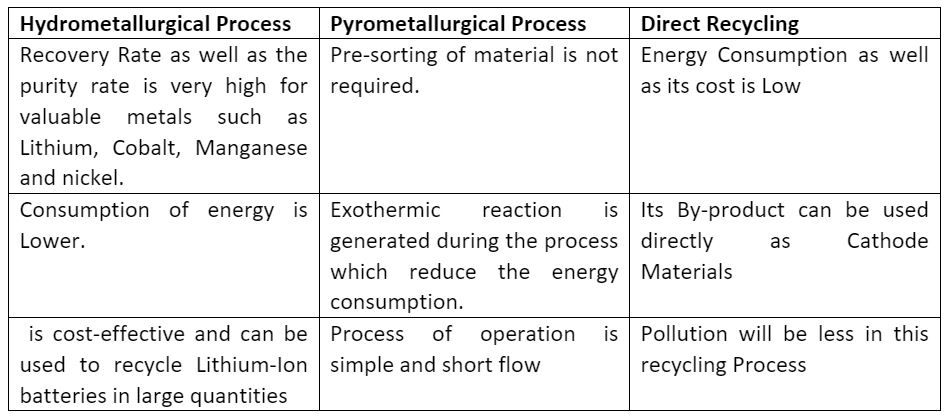
Disadvantages
4. Challenges and opportunities
Each process has its own unique set of challenges and opportunities which can be looked at and there is a need for future improvement.
Hydrometallurgy Process
This Process requires a considerable amount of water, which can be a challenge in areas with limited water resources. Additionally, the process produces a significant amount of waste, which must be disposed of properly.
The opportunity of this process is that it can achieve high metal recovery efficiency from spent Lithium-ion batteries. Also, this process can be used to recover specific metals depending on the composition of the spent batteries.
Pyrometallurgy Process
The high temperatures required for this process can lead to significant energy consumption and environmental emissions. Additionally, the process can be challenging to control and may result in the loss of valuable metals.
The opportunity in this is that the recovered metals can be used to produce new batteries or other products, reducing the dependence on primary sources of Cobalt, Nickel and Lithium metals.
Direct Process
This process requires significant investment in research and development to optimize the recycling process and ensure the safety and performance of the recycled components.
CONCLUSION
The recycling of lithium-ion batteries is essential to reduce the environmental impact of battery production, recover valuable metals and ensure the availability of critical metals for future generations. There are several recycling processes available, including hydrometallurgy, pyrometallurgy, and direct recycling, each with its own unique set of challenges and opportunities.
In the future there is a possibility of optimizing the different processes to improve the extraction efficiency and reduce the consumption of chemicals. There is also a scope in exploring the use of environmentally friendly solvents or reagents to make the process more sustainable. It could also focus on developing advanced technologies that can recover metals from the spent batteries more efficiently and effectively.
Overall, the development of efficient and sustainable recycling processes for lithium-ion batteries is crucial. Recycling facilities must consider the specific needs and constraints of their operations to determine the optimal recycling process. Through continued research and development, the recycling of lithium-ion batteries can become an even more efficient and sustainable process, contributing to a cleaner and more sustainable future.
